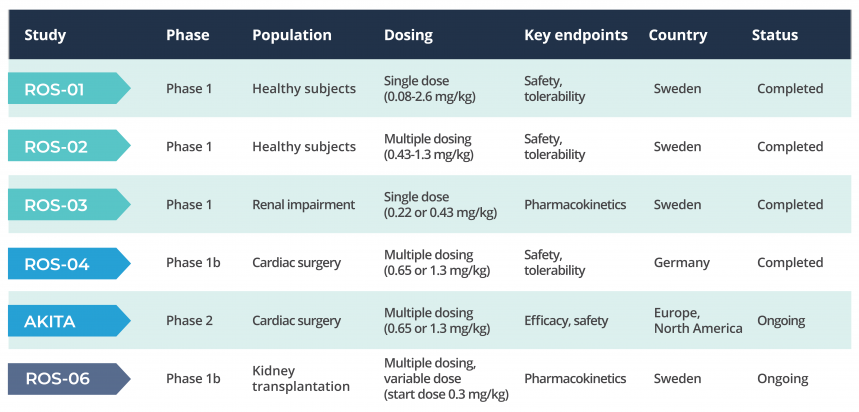
Clinical studies in cardiac surgery
ROS-04
On the basis of existing phase 1 results the first study in patients undergoing cardiac surgery (phase 1 b) was initiated in early 2021. The study was conducted at a larger site for cardiothoracic surgery in Germany. The main purpose of the phase 1b study was to evaluate the investigational drug’s safety and tolerability profile and its pharmacokinetic properties in 12 patients who underwent extensive open-chest heart surgery and had additional risk factors for acute kidney injury (AKI). The study results showed that RMC-035 was well tolerated and no side effects attributed to the investigational drug were reported. Pharmacokinetic analyses showed desirable blood concentrations of RMC-035, providing support for the dosing regimen intended to be used in the planned phase 2 study.
In addition, explorative analyses of several well-established biomarkers indicate that RMC-035 potentially has the ability to protect kidney cells from injury associated with heart surgery.
AKITA study
In early 2022, a global Phase 2 study of RMC-035, the AKITA study, was launched. The primary purpose of the phase 2 study is to demonstrate a clinically relevant treatment effect in the target patient population, i.e. patients at increased risk for developing acute kidney injury (AKI) after open chest cardiac surgery. A number of important secondary endpoints, such as changes in renal function during hospitalization and up to three months after surgery, will be analysed. The study design will facilitate an informed decision about optimal Phase 3 design. The design of the phase-2 study has been anchored with leading global experts in the field.
The AKITA study is being conducted in both North America and Europe and includes approximately 270 patients. The phase 2 study is randomized, double-blind and placebo-controlled and enables, in the event of a positive outcome, the start of a pivotal phase 3 study.